A Semiconductor is a type of material which acts as an insulator and as a conductor at specific condition. This type of material very important as it plays vital role in the field of electronics, communication, and control circuits. Before further discussion about what is semiconductor, it’s construction and types, we need to know classification of materials on the basis of electrical conductivity.
Classification of Materials On The basis of Electrical Conductivity:
Before going for classification of material on the basis of electrical conductivity we must have knowledge of energy band. According to Neal Bohr’s Model, electrons are presents in shells around the nucleus.
Example, carbon of atomic number of 6 so we can write atomic configuration as, 1s^2, 2s^2, 3p^2.
Whenever chemical bond is formed only electrons presents at the outermost orbit, become a part of the bonding. Now suppose, two atoms bring close to each other to form a bond, within atom there is electrostatic attraction between electron and nucleus, when two atoms come close to each other there is the force of attraction between electrons and nucleus of two different atoms and repulsive force between two electrons of two atoms.
When bond is formed energy level of electrons will change. At each energy level there are electrons existing which splits in to two different levels due to bonding are as anti-bonding molecular orbital and bonding molecular orbital. Anti-bonding molecular orbital have higher energy than bonding molecular orbital. When number of atoms gets together there are different energy levels creates energy bands of different energy level. The energy band of high energy called conduction band and other is called valence band.
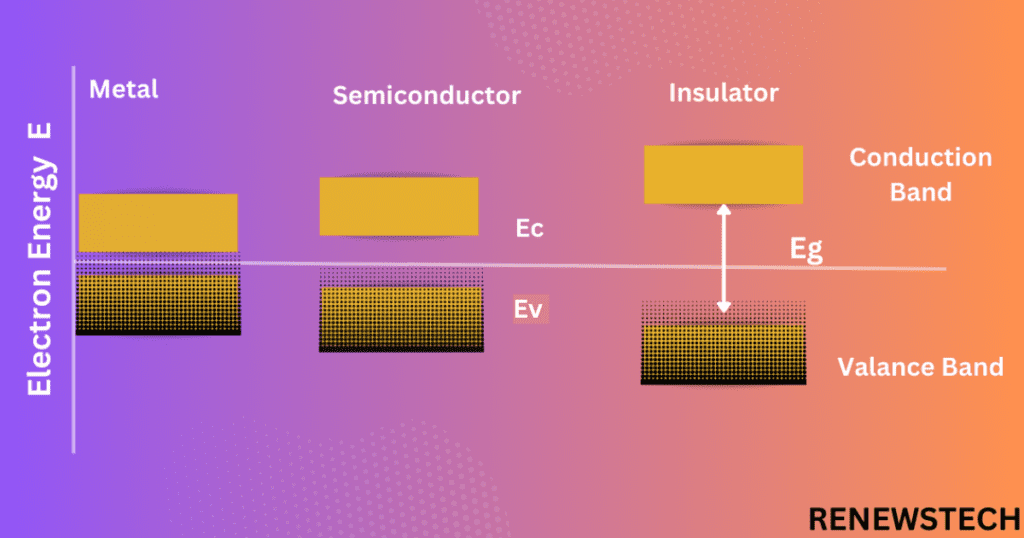
Valance Band:
It is the collection of energies of valance electrons which presents at outermost shell but at 0 Kelvin temperature these are tightly bound to the nucleus by means of electrostatic force and not ionized so these lies in valance band thus no current will flow. If these electrons get free from nucleus, they will start conduct the current and these are now lies at conduction band.
Conduction Band:
This is the band of energy of only electrons free from nucleus. As temperature increases, electrons at outermost shell gets energy and they will ionize from nucleus and moves from valence band to conduction band, thus current will flow.
Conductor
In conductors valance band and conduction band are overlapped that means there is no energy gap between two bands. There are number of electrons ionized from nucleus and required less energy to move free electrons from valance band to conduction band.
Conductors are the materials which having large number of free electrons, when a potential applied to conductor currents starts flowing through it.
Atoms easily give ups and become positively charged. As soon as voltage is applied to the conductor, an electric field is created that forces free electrons and thus producing current.
Insulator
In an insulator there is large energy gap between valance band and conduction band, thus large amount of energy i.e., greater than 5eV is required to move electrons from valance band to conduction band by which reduce the energy gap between two bands.
These materials have very few free electrons and hence very small electrons flows through it, which we ignore such small current and assume that insulator.
Insulators also called as dielectric material.
Examples: wood, mica, diamond, air, paper, plastic etc.![]()
![]()
![]()
![]()
Semiconductors
In semiconductor there is small energy gap between energy bands, thus small amount of energy is required to ionize electrons from nucleus so they will move from valance band to conduction band
These are materials in which number of free electrons per volume lie between conductors and insulators i.e.10^6, to 10^21 . Thus, we can control number of free electrons, so we can even control conductance and resistance
Number of free electrons can be controlled and hence conductivity can be varied as per requirement by means of controlling temperature, light and by means of doping.
Examples: Silicon Si, Germanium Ge, Gallium Arsenide GaAs, Indium Phosphorous InP, etc
Classification of Semiconductor:
1.Pure Semiconductor
A semiconductor in which no impurities added. A pure semiconductor is a semiconductor of perfect crystal structure which is practically not possible.
Hole: when an electron which are ionized from nucleus i.e., free electrons moves from valence band to conduction band there will vacancy of free electrons in valence band called as hole. Thus, electron in conduction band and hole in valence band created an electron- hole pair.
2.Intrinsic Semiconductor
In this semiconductor, no impurities added but crystal defect in structure. Valence band have high energy than conduction band, to move electrons from valence band to conduction band we provide some amount of energy on the other hand to move electrons from conduction band to valence band we can get energy.
Thermal equilibrium:
when an electron moves from valance band to conduction band then number of holes in valence band is equal to number of free electrons came from valence band called as generation of electron-hole pair and on the other hand if electrons move from conduction band to valence band there is recombination of electron- hole. Therefore, “the state at which concentration of electron and hole in conduction band and valence band and conduction band are equal during the generation of electron-hole pair and recombination of electron-hole this condition is called as thermal equilibrium”.
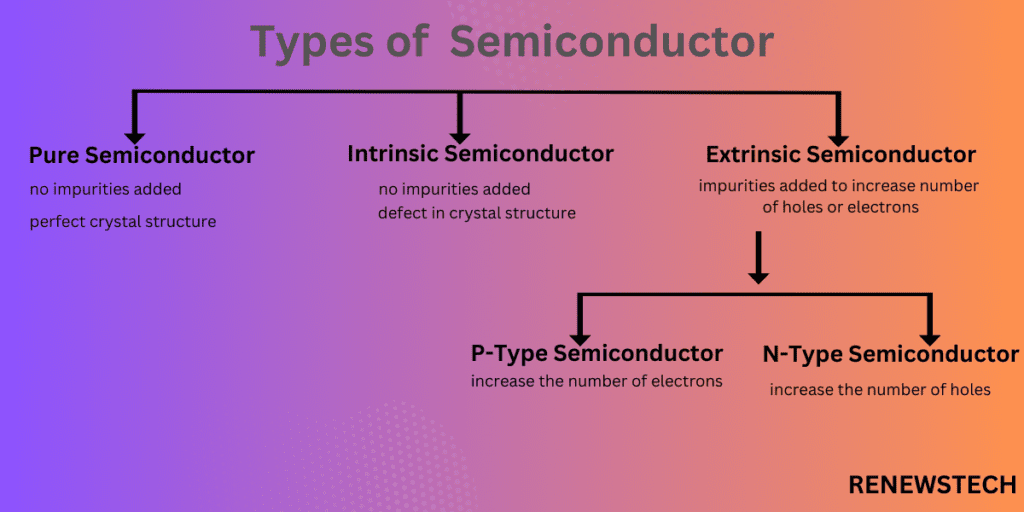

3.Extrinsic Semiconductor:
A semiconductor in which impurities are either to increase number of holes or electrons. Also, there is a crystal defect in structure of extrinsic semiconductor.
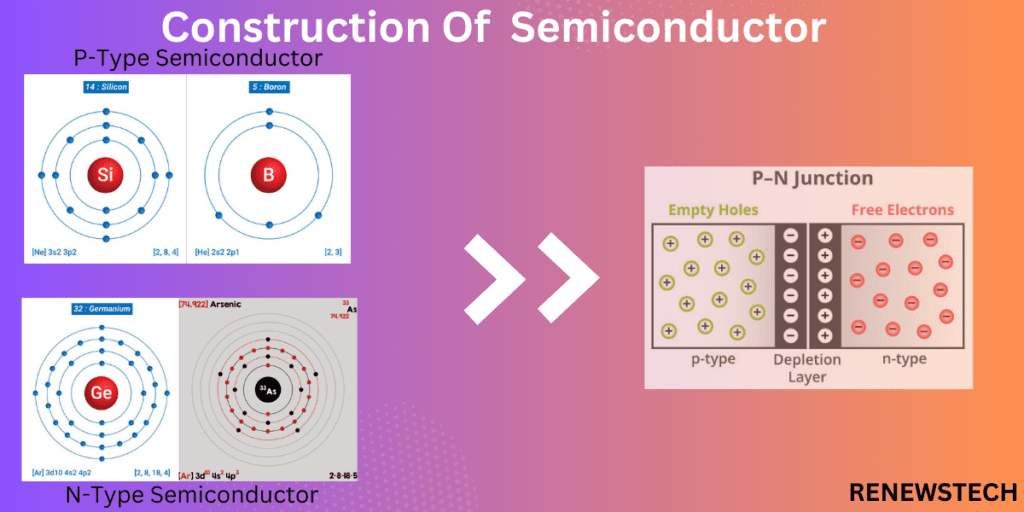
P-type semiconductor:
A semiconductor in which impurities are added to a pure semiconductor to increase concentration of electrons called as P-type semiconductor. To increase the number of electrons, impurities phosphorus from group V which is pentavalent impurity added to silicon or germanium from group IV.
N-type semiconductor:
A semiconductor in which impurities are added to a pure semiconductor to increase concentration of holes called as N-type semiconductor. To increase the number of holes, impurities boron from group III which is trivalent impurity added to silicon or germanium from group IV.
Construction of Semiconductor